 Overview
Overview
In 2006, the introduction of a highly effective vaccine against human papillomavirus (HPV) exposed the need for population-based surveillance of HPV-related diseases. In 2007, the Centers for Disease Control and Prevention funded a collaboration between CEIP and the California Department of Public Health STD Control Branch to conduct surveillance of cervical pre-cancer including cervical intraepithelial neoplasia (CIN) 2/3 and adenocarcinoma in situ (AIS). This surveillance effort is ongoing in Alameda County, California in partnership with the Alameda County Department of Public Health and the California Cancer Registry.
Objectives
- Determine the burden of CIN 2/3, AIS, and cervical cancer in Alameda County and monitor trends in incidence
- Conduct HPV testing for a subset of cases to monitor HPV types
- Monitor and evaluate the impact of the HPV vaccines
Laboratory Reporting
High-grade cervical pre-cancer and cervical cancer is reportable in Alameda County. Please see additional information on reporting eligible cases.
In the News
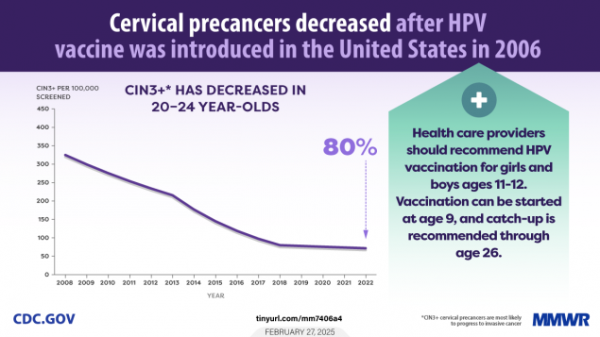
- Just published: Trends in Cervical Precancers Identified Through Population-Based Surveillance in February 27, 2025 Morbidity and Mortality Weekly Report (MMWR)
 International HPV Awareness Day and the “One Less Worry” campaign offers resources and social media for patients, providers, and partners
International HPV Awareness Day and the “One Less Worry” campaign offers resources and social media for patients, providers, and partners- Get yourself tested for STI’s with a free at-home kit!
- Make history and eliminate cervical cancer for ever, urges WHO chief
Cervical cancer is the second most common cause of cancer death in reproductive-aged women globally, according to the UN health agency, yet preventable with vaccination and screening that includes appropriate follow-up and treatment. Read more about the Global Strategy to Accelerate the Elimination of Cervical Cancer. - Principal Investigator Dr. Ina Park’s book Strange Bedfellows: Adventures in the Science, History, and Surprising Secrets of STDs is available online and in most bookstores. Listen to interviews from her virtual book tour here.
Infographic
National Collaborating Surveillance Sites
- Connecticut Emerging Infections Program, Yale University
- Tennessee Emerging Infections Program, Tennessee Department of Health, Vanderbilt University
- New York Emerging Infections Program, University of Rochester
- Oregon Emerging Infections Program, Oregon Health Authority
Questions?
Please contact:
- Ina Park, MD MS
Principal Investigator
Associate Professor, UCSF School of Medicine
ina.park@ucsf.edu - Erin Whitney, MPH
HPV Surveillance Project Coordinator
STD Control Branch, California Department of Public Health
850 Marina Bay Parkway, Bldg P, 2nd Floor
Richmond, CA 94804
erin.whitney@cdph.ca.gov
(510) 620-2379
Links
For more information on HPV and the HPV vaccine:
- The CDC’s HPV-Impact Page
- The American Sexual Health Association’s HPV Website
- Published HPV-Impact articles on PubMed.gov
- The Centers for Disease Control and Prevention HPV Page
- California Department of Public Health Human Papillomavirus Page
- Advisory Committee on Immunization Practices (ACIP) Recommendations for the HPV Vaccine
- CDC’s Adolescent HPV Vaccination Coverage Dashboards
